Address
MaRS Center, South Tower
101 College Street, Suite 300
Toronto, ON, M5G 1L7
Get in touch
(416) 673-8170
Our Science
Molecular Design Meets Material Science
At Ripple, we discovered that with a small amount of chemical engineering, drugs can be transformed into implantable prodrug materials.
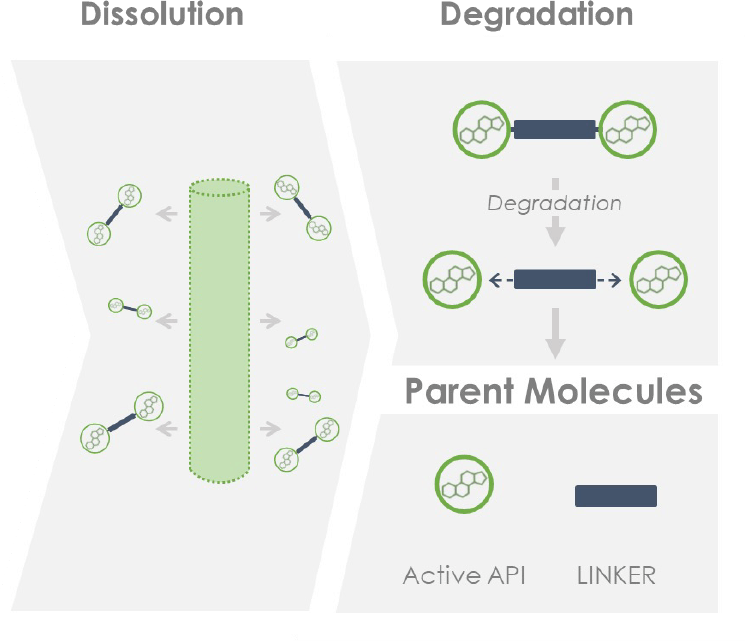
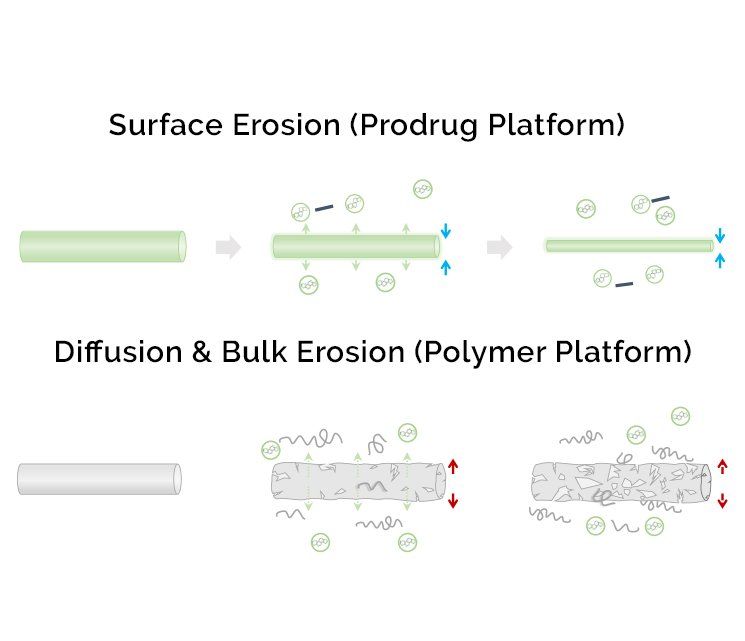
These remarkable materials undergo surface erosion which results in release of the active drug.
By tuning surface area and implant geometry, drug dose and duration can be precisely designed to meet the therapeutic need.
The proprietary Epidel® prodrugs have unique properties that allow them to be processed into standalone drug delivery implants (e.g. intravitreal implants, micro/nanoparticles, etc.) or as coatings on medical devices.
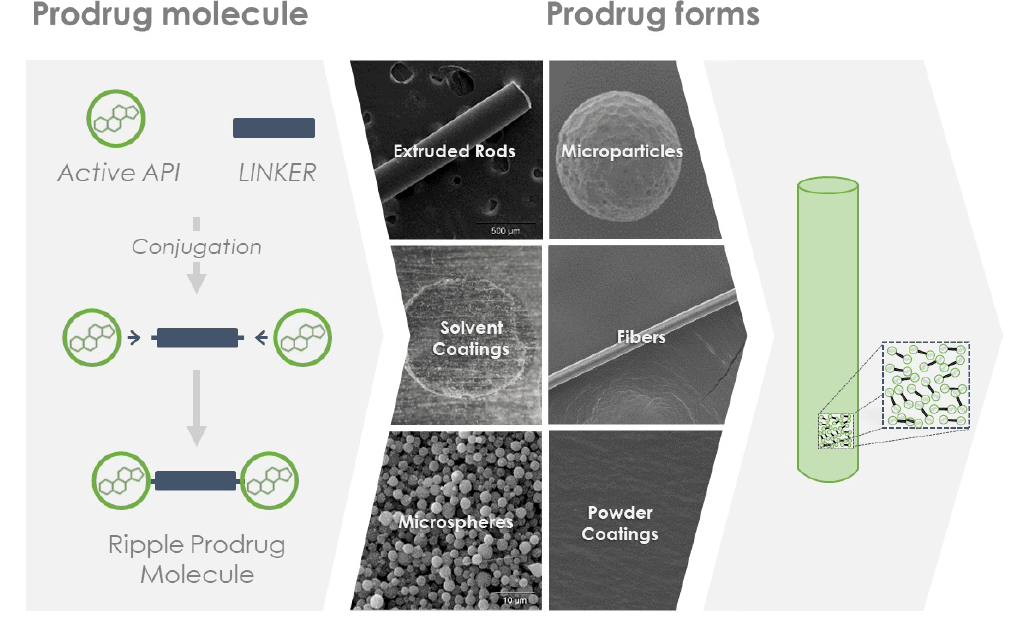
Engineering drugs that deliver themselves
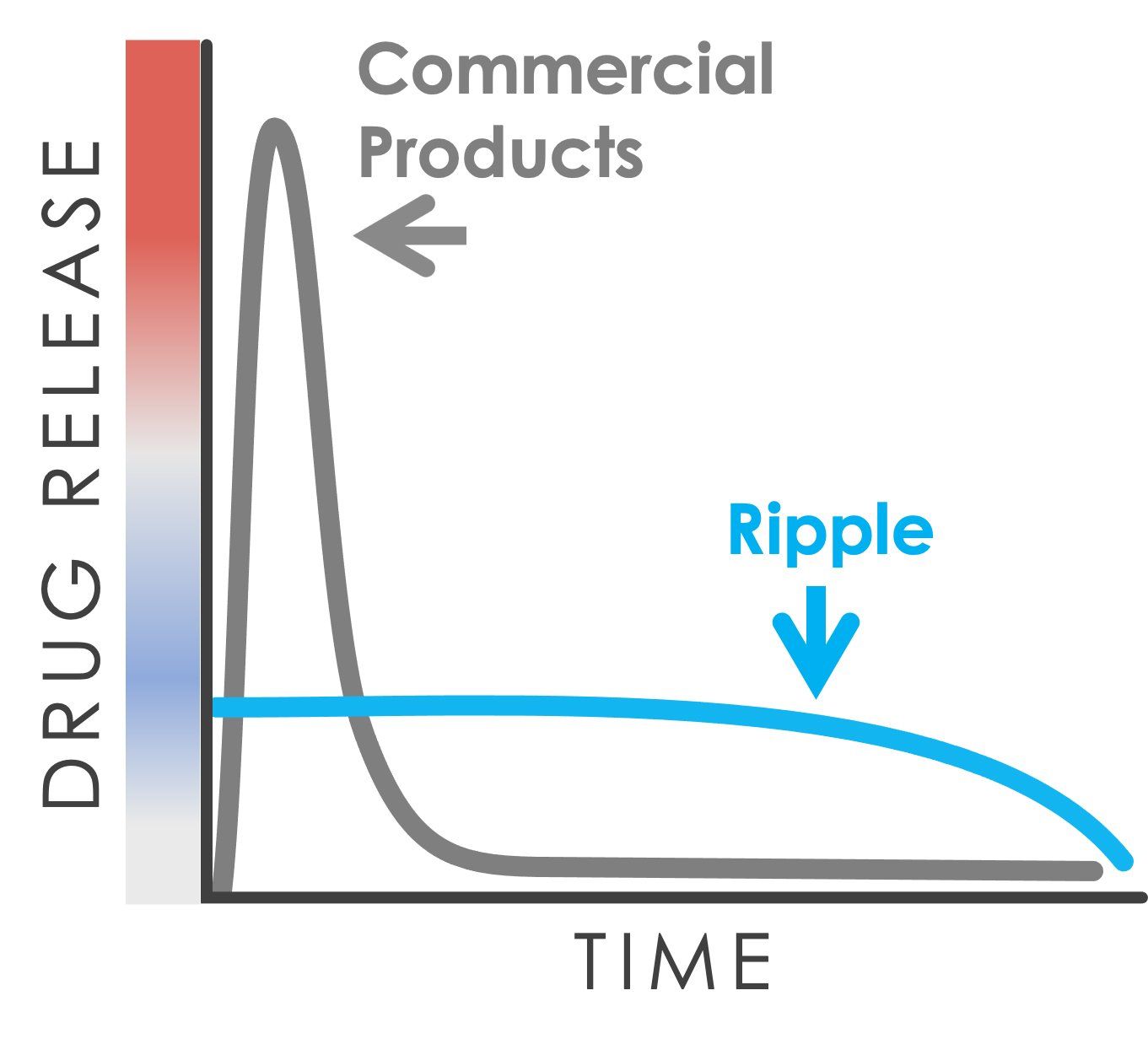
Drug dissolves from the surface of the Epidel implant via surface erosion in a highly predictable manner: dose is dictated by implant surface area; dose duration by implant diameter.
The result is an ability to finely tune pharmacokinetics to meet the needs of a target indication with precision control of drug dosing without the bulk added by a polymer.
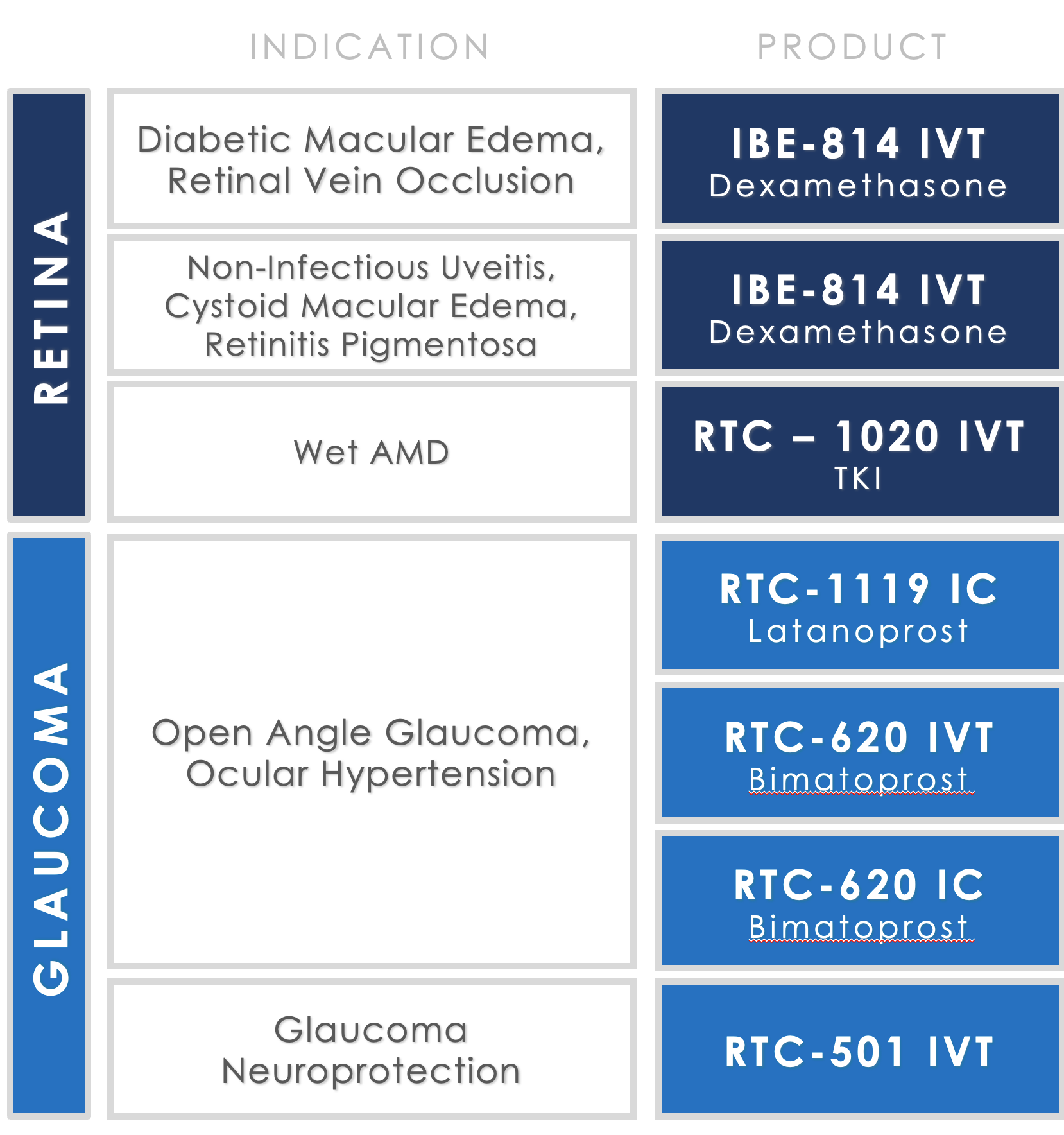
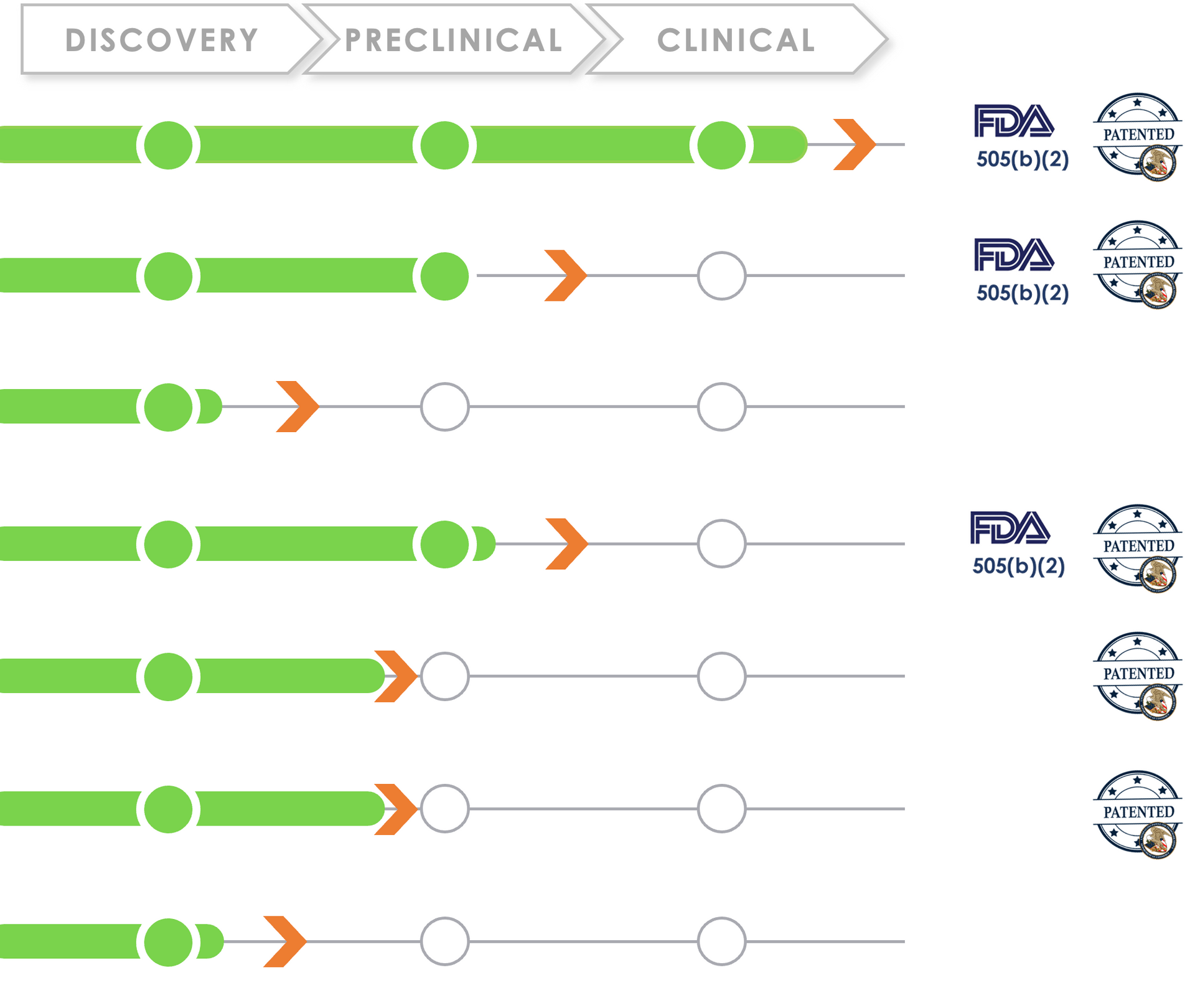
Pipeline
Ripple Therapeutics ophthalmology pipeline addresses multiple $1B+ market opportunities
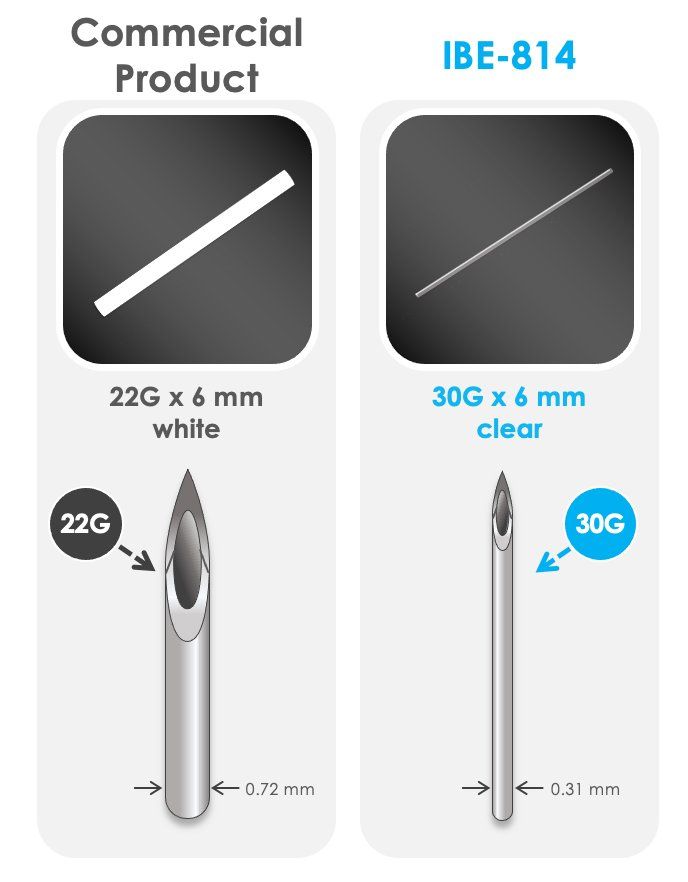
IBE-814 IVT dexamethasone releasing implant
IBE-814 IVT is aimed at being best-in-class treatment of posterior inflammatory eye diseases, including diabetic macular edema (DME), retinal vein occlusion (RVO) and non-infectious uveitis (NIU).
Target Product Profile

Competitive advantage over IVT steroid market leader
Potential for ↓ in steroid-related cataract & IOP AEs
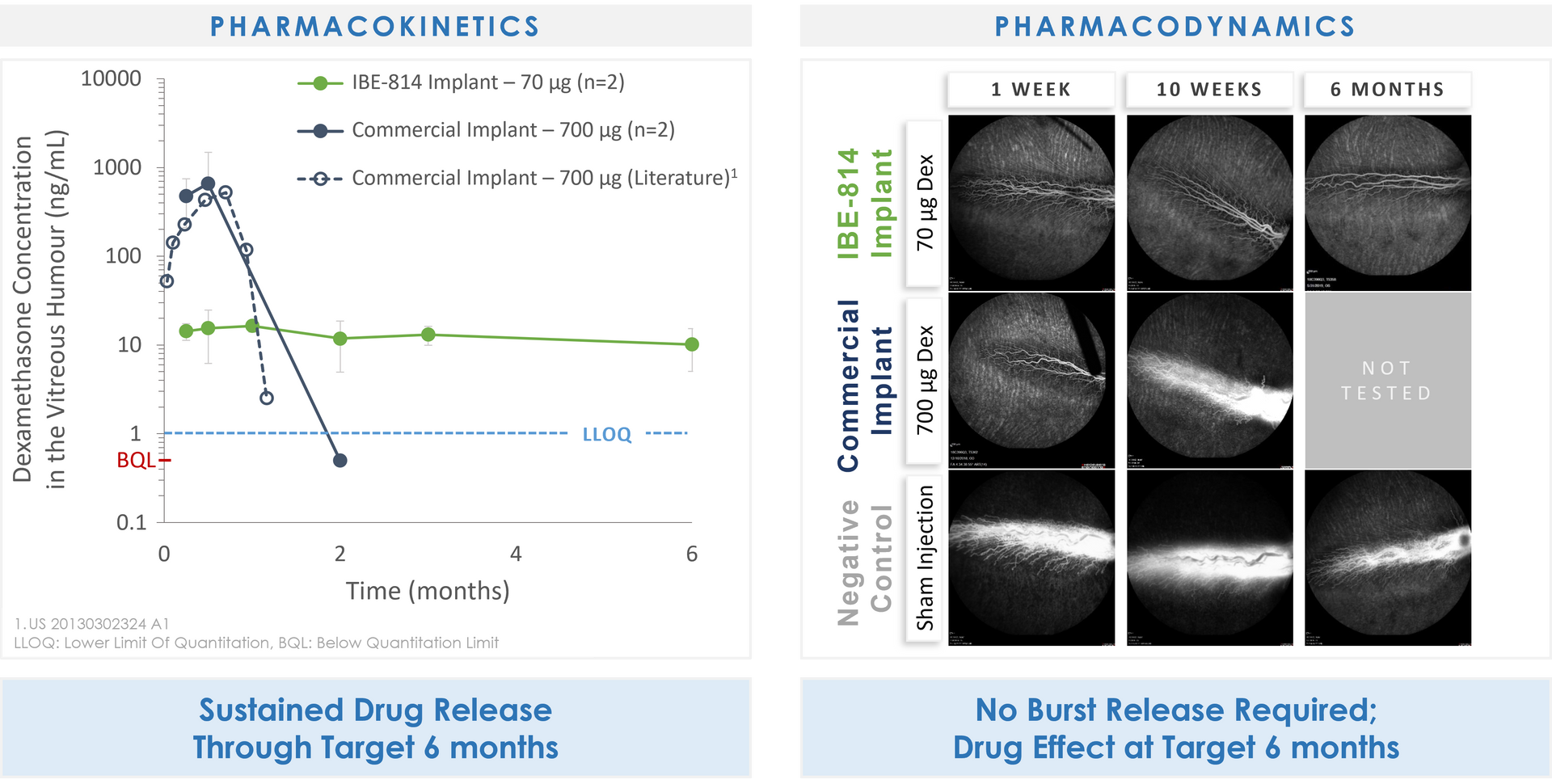
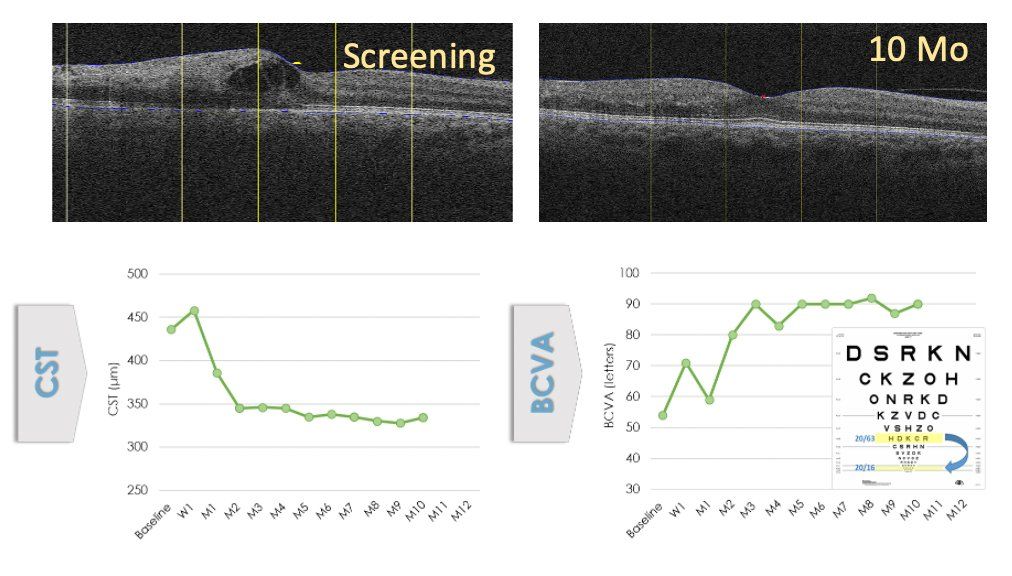
IBE-814 IVT Preclinical to Clinical Translation – Efficacy
•Reducing edema, improving vision, extending duration of clinical benefit
•Surface erosion drug delivery enables precision control of dose and duration
Preclinical

CLINICAL
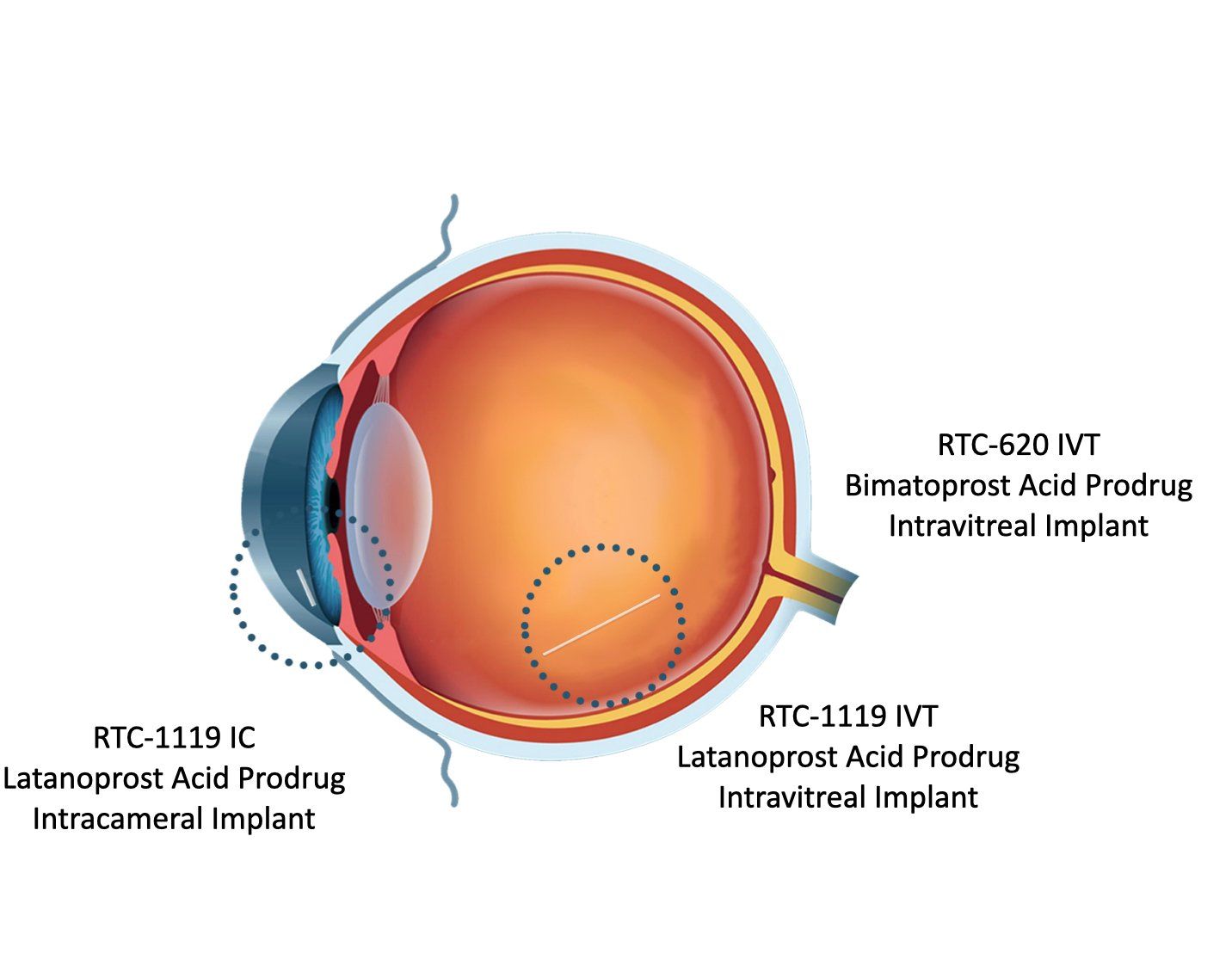
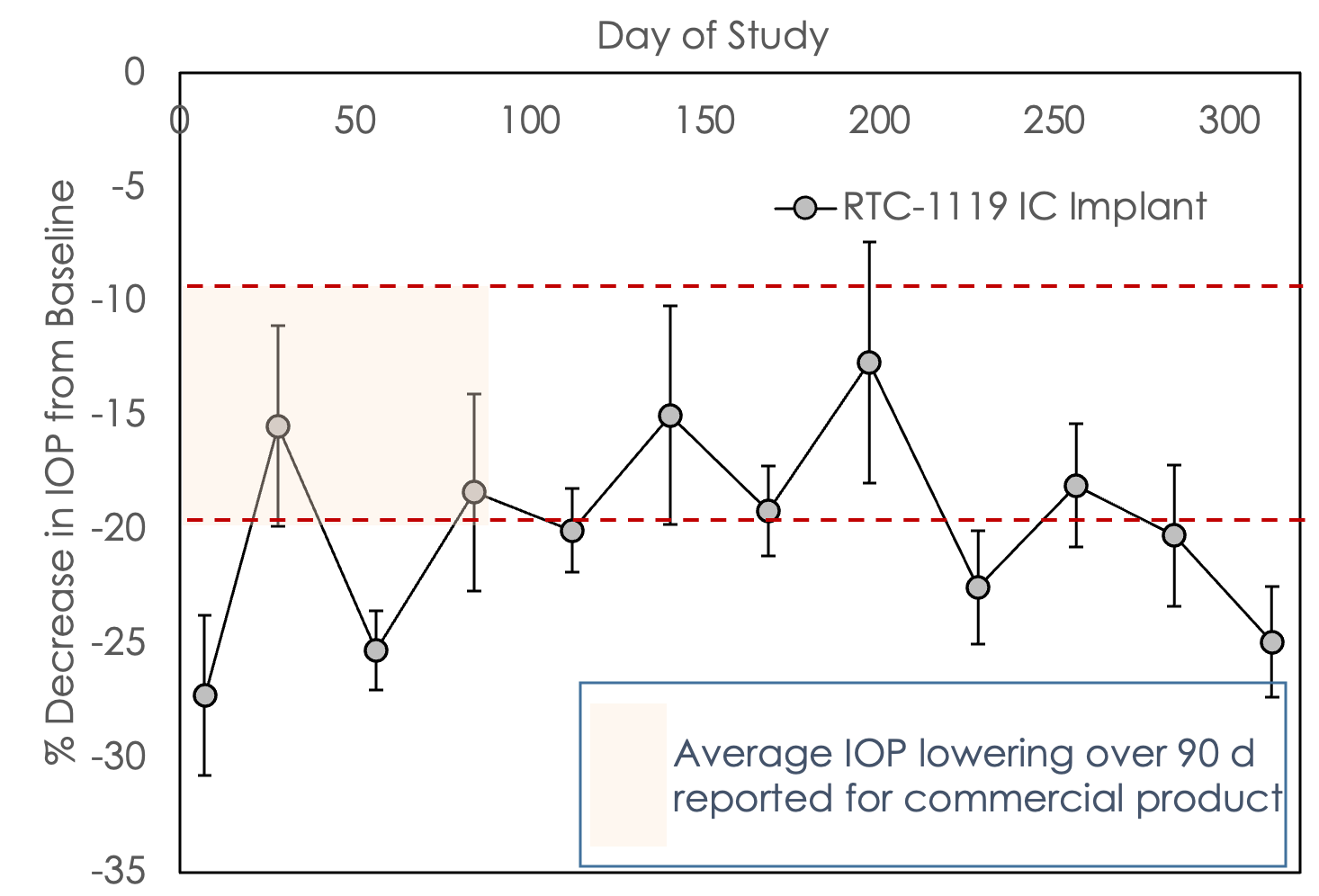
RTC-1119 implant designed to repeatably treat Open Angle Glaucoma and Ocular Hypertension
Target Product Profile

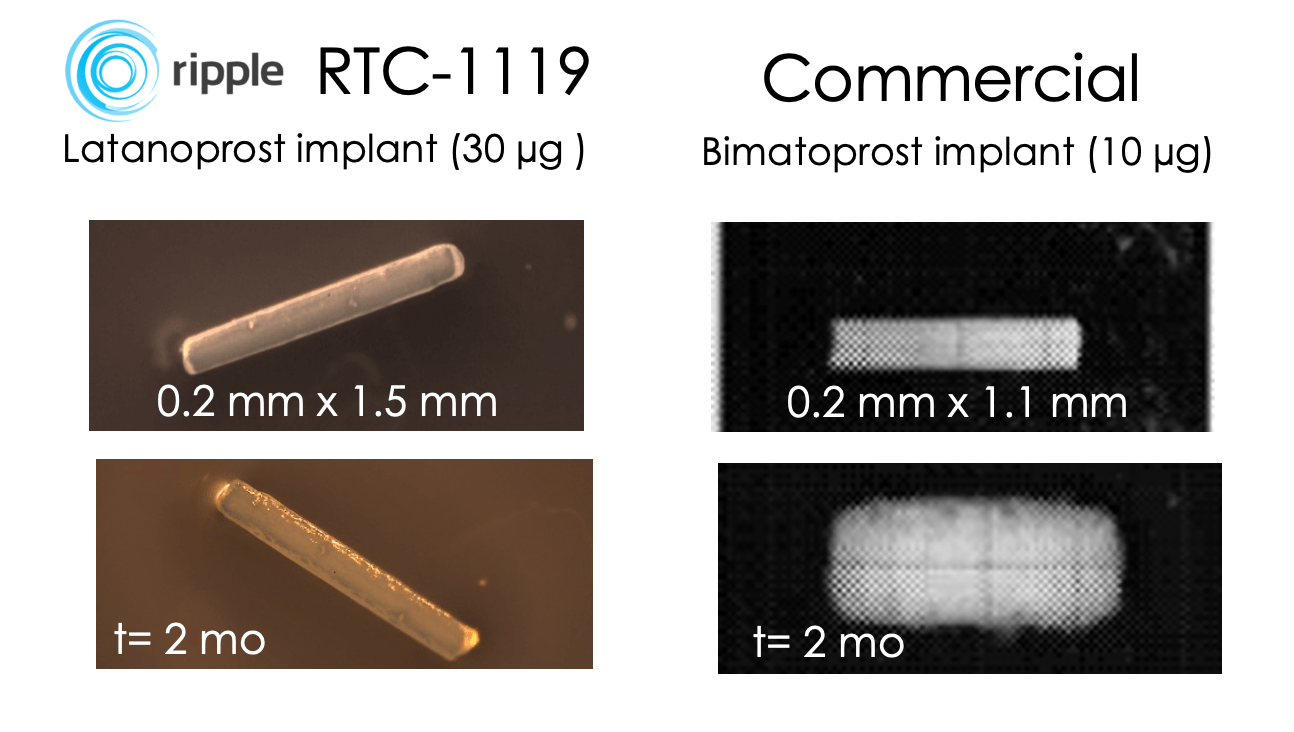
RTC-1119: Retreatment every 6-12 months
•Small, non-swellable implant prevents corneal contact
•Implant no longer present after drug has been released
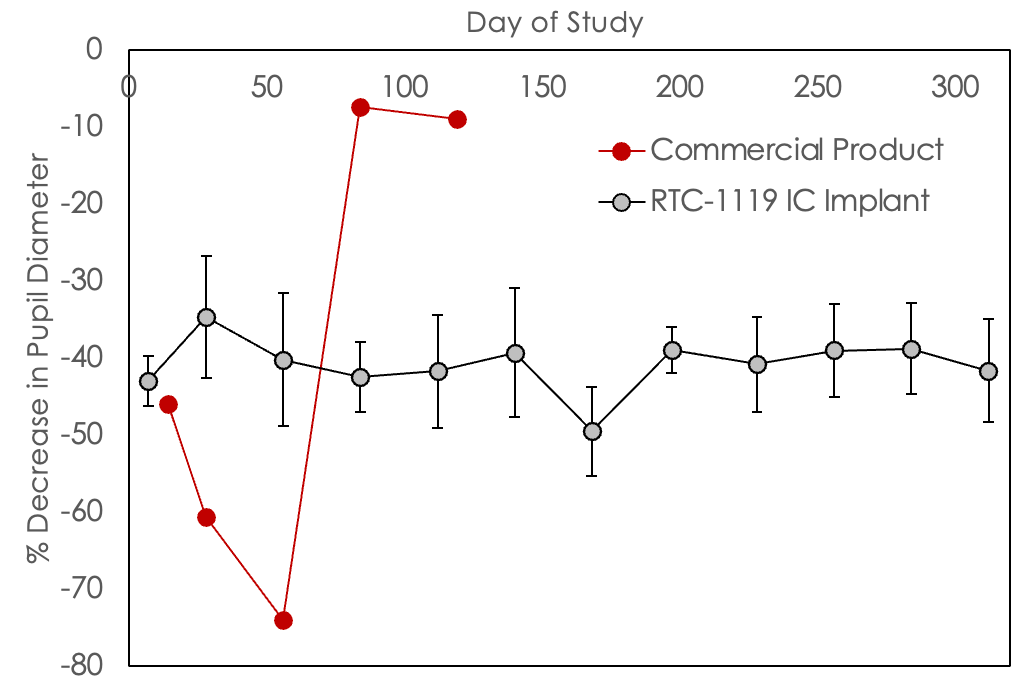
Glaucoma: RTC-1119 IC preclinical demonstration of durability and effect
IOP
Pupil Diameter